Abstract
Background:
DDX41 gene is located on chromosome 5q35 and is believed to be a tumor suppressor gene involved in splicing of mRNA. Mutated DDX41 have been identified as germline mutation (m) in families with cases of myeloid neoplasm and have shown to cause acquisition of other somatic mutations resulting in MDS/AML. At the same time, somatic pathogenic DDX41 mutations are known to occur. Variant of unknown significance (VUS) is an intermediate tier between benign and pathogenic (path) mutations (hence uncertain) and can be re-classified based on their clinical impact. In this report we analyzed the clinical impact and relevance of DDX41VUS in patients (pts) being evaluated for myeloid disorders.
Methods:
We reviewed 4,524 consecutive pts who underwent NGS (MC OncoHeme 42 genes panel) testing between 2018 and 2021 performed on cases of suspected or known myeloid disorders. We identified 58 (1%) consecutive pts with DDX41VUS, among them one pt had proven concurrent DDX41 germline VUS and 7 pts with DDX41VUS had concurrent DDX41path mutation. We analyze clinical characteristics of pts with DDX41VUS and compared it with cohort of 32 DDX41path mutated pts.
Results:
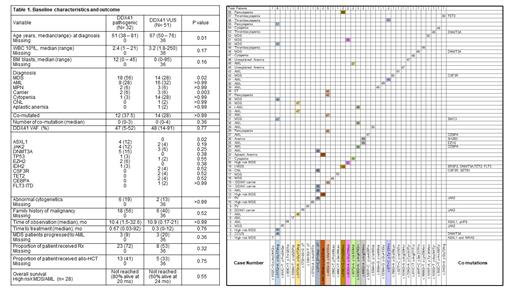
Baseline characteristics are summarized in Table 1, and Figure 1 illustrates DDX41VUS per case with variant allele frequency (VAF) and observed concurrent mutations. The median VAF for DDX41 VUS was 48 % (range, 14-91%). Co-mutations were found in 14 (28%) pts with VUS, and the reminder (n=44, 72 %) had isolated DDX41VUS. Among them AML (16 [32%]), MDS (14 [28%]), cytopenia (14 [28%]) MPN (3 [6%]), CNL (1 [2%]) and aplastic anemia (1 [2%]) were the commonly occurring myeloid disorder (Table 1, Figure 1). Eleven of 14 pts with isolated cytopenia had isolated DDX41VUS. Conversely, among 15 evaluable pts with isolated DDX41VUS, 13 (87%) pts had normal cytogenetics and 40% of pts had family history of solid or hematological malignancies. We observed a significant difference in hematologic diagnoses: DDX41VUS patients were significantly less often diagnosed with MDS or CCUS, and more often associated with cytopenias NOS.
Recurrent, non-random DDX41VUS sequences were observed; most frequent occurring amino acid change among the 58 pts were Gly173Arg (n=6), Met155lle (n=5), Pro258Leu (n=3) Arg479Gln (n=3), Val303Met (n=2), Lys331del (n=2), Arg479His (n=2) and Tyr33His (n=2) (Figure 1). 5 of 6 pts with Gly173Arg had isolated DDX41VUS, and among them 4 were diagnosed with MDS suggesting this could be a clonal event, and possible driver mutation, notable in Figure 1. The median overall survival (OS) of pts with high risk MDS/AML harboring DDX41VUS was not reached (60% alive at 2 years). Between pts with DDX41path and DDX41VUS, proportion of pts with co-mutations (p >0.99), cytogenetic abnormalities (p >0.99), family history of malignancies (p 0.52), time to treatment (p >0.99), MDS progression to AML (p 0.36) and OS (among high risk MDS/AML pts) (p 0.55) was not significantly different, suggesting that phenotypic features of DDX41VUS are similar to DDX41path , and that both carry a similar, more indolent prognosis (Table 1).
Conclusion:
Our reports demonstrated that DDX41VUS occurs in 1% of patients with myeloid disorders and shares distinct diagnostic features but comparable prognostic features vs. DDX41path, including family history of hematological malignancies. It is possible that many of the cases with 'cytopenias' and DDX41VUS would meet criteria for CCUS if the variant was isolated classified as pathogenic, confirming the importance of extending our understanding of DDX41 variants. Further analysis to characterize the biological impact of DDX41VUS is underway.
Badar: Pfizer Hematology-Oncology: Membership on an entity's Board of Directors or advisory committees. Al-Kali: Novartis: Research Funding; Astex: Other: Research support to institution. Patnaik: Kura Oncology: Research Funding; Stemline Therapeutics: Membership on an entity's Board of Directors or advisory committees; Stemline Therapeutics: Membership on an entity's Board of Directors or advisory committees. Litzow: Astellas: Research Funding; Omeros: Other: Advisory Board; Jazz: Other: Advisory Board; Pluristem: Research Funding; Amgen: Research Funding; Biosight: Other: Data monitoring committee; Actinium: Research Funding; AbbVie: Research Funding. Foran: boehringer ingelheim: Research Funding; abbvie: Research Funding; takeda: Research Funding; trillium: Research Funding; revolution medicine: Honoraria; bms: Honoraria; servier: Honoraria; novartis: Honoraria; pfizer: Honoraria; aptose: Research Funding; taiho: Honoraria; certara: Honoraria; sanofi aventis: Honoraria; syros: Honoraria; actinium: Research Funding; gamida: Honoraria; OncLive: Honoraria; kura: Research Funding; h3bioscience: Research Funding; aprea: Research Funding; sellas: Research Funding; stemline: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal